When you’ve had a kidney, liver, or heart transplant, your life changes in ways no one prepares you for. One of the biggest shifts? Taking the same pills every single day - not because you feel sick, but because your body might otherwise reject the new organ. Two drugs make that possible: cyclosporine and tacrolimus. Both are powerful, both are essential, and both come with a hidden risk: switching between generic versions can be dangerous.
Why These Two Drugs Are So Different
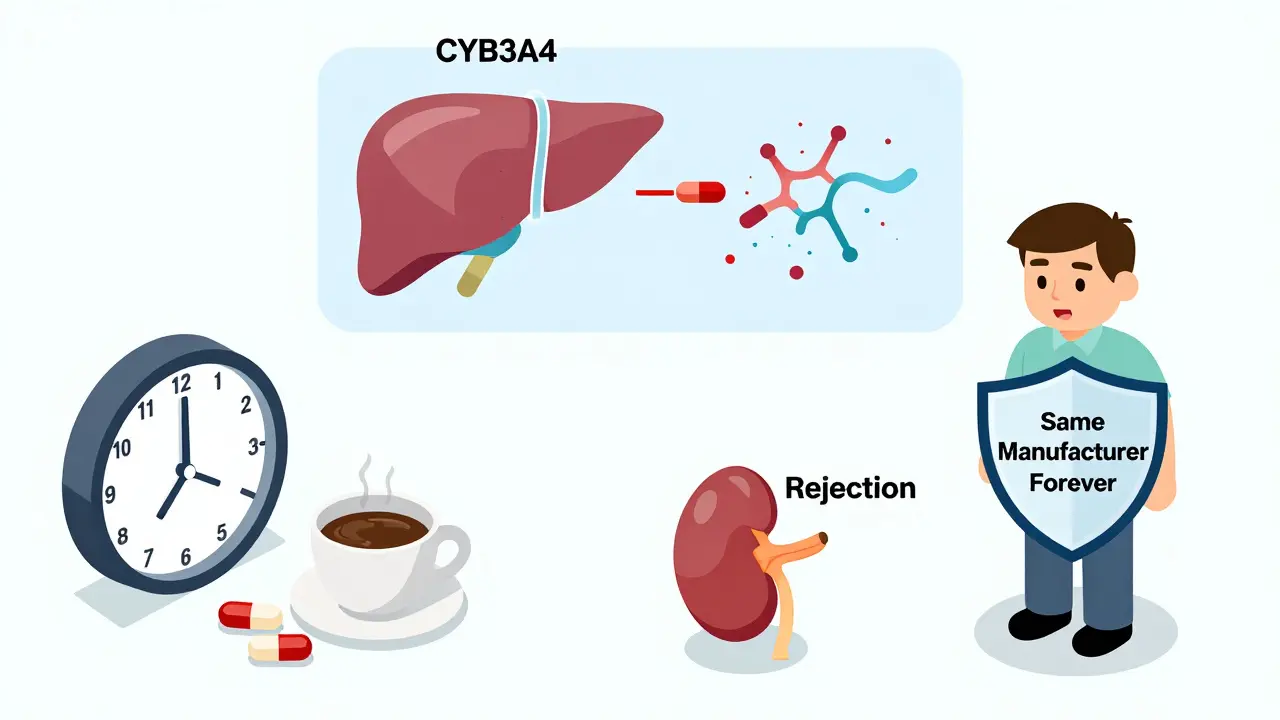
Cyclosporine and tacrolimus work the same way - they block a protein called calcineurin, which tells your immune system to attack the transplanted organ. But that’s where the similarities end. Tacrolimus is about 20 to 100 times more potent. You take 5 mg of it twice a day. For cyclosporine? You take 150 mg twice a day. That’s a huge difference in pill count, but even more important: the blood levels doctors track are completely different. For tacrolimus, the safe range is 5-15 ng/mL. For cyclosporine? 100-200 ng/mL. Miss by just a few points on tacrolimus, and your body might start rejecting the transplant. Go too high, and you risk kidney damage or seizures. Both drugs are metabolized by the same liver enzyme (CYP3A4), so anything that affects it - grapefruit juice, certain antibiotics, even some herbal supplements - can throw your levels off. But tacrolimus is more sensitive. A small change in how your body absorbs it can mean the difference between safety and crisis.The Generic Switch Problem
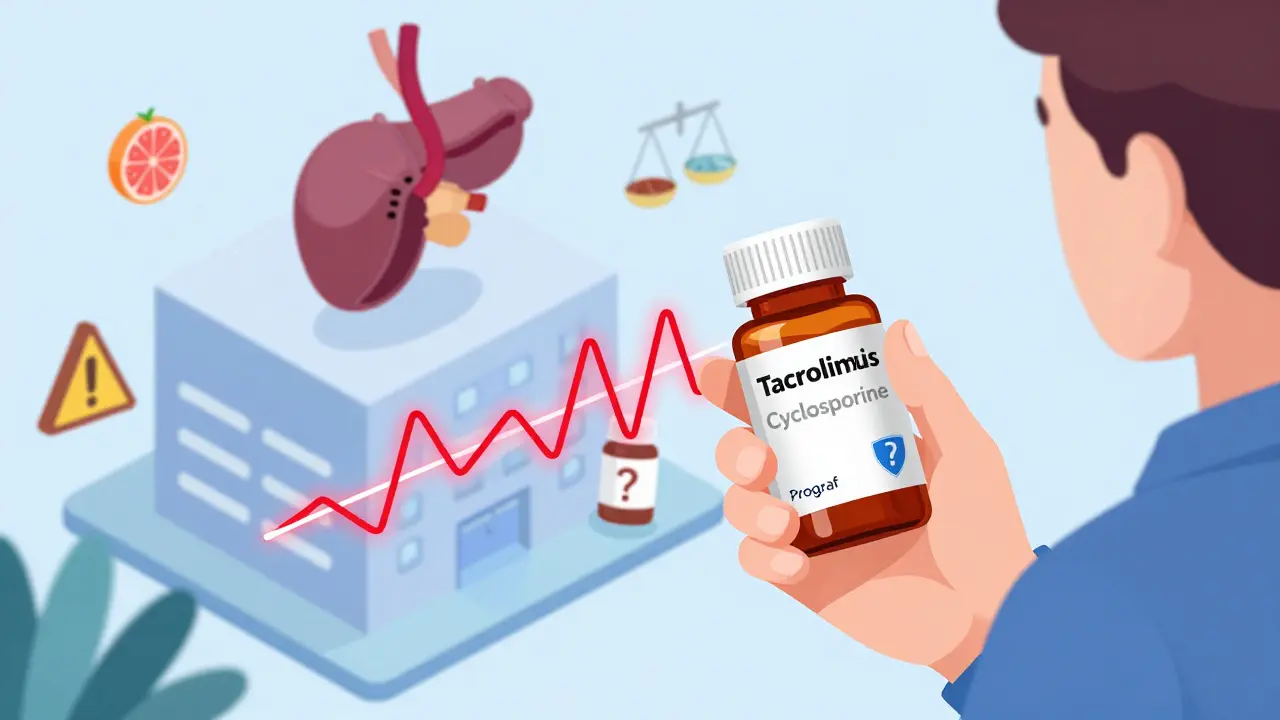
Brand-name tacrolimus (Prograf) used to cost over $1,200 a month. Now, generic versions cost $300-$500. Cyclosporine went from $800 to $150-$300. That’s a lifesaver for patients on fixed incomes. But here’s the catch: these aren’t like switching from one brand of ibuprofen to another. Generic drugs must prove they’re “bioequivalent” - meaning they deliver the same amount of drug into your bloodstream as the brand name, within a 80-125% range. Sounds fair, right? But for drugs with a narrow therapeutic index like these, that 45% window is huge. Two generics can both be “approved,” yet one might release the drug faster, causing a spike in blood levels. Another might release it slowly, leaving you underdosed for hours. Real patients have paid the price. One Reddit user, u/KidneyWarrior, switched from Prograf to a generic and saw their tacrolimus level drop from 8.5 to 5.2 ng/mL in two weeks. That’s below the minimum safe level. They ended up hospitalized with a mild rejection episode. Another person said their nephrologist refuses to let them switch to generic cyclosporine because their levels were too unstable the first time. A 2022 survey of over 1,200 transplant patients found that 42.7% noticed side effects change after switching to a generic. Nearly 1 in 5 needed a dose adjustment because their blood levels went haywire. And it’s not just perception - the U.S. Renal Data System showed medication non-adherence is 15.3% higher among people taking generics, partly because they don’t trust them.Why Not All Generics Are Created Equal
There are 14 FDA-approved generic versions of tacrolimus from eight different manufacturers. Eleven generics exist for cyclosporine. Each has a slightly different filler, coating, or oil base. For cyclosporine, the old version (Sandimmune) was an oil-based capsule that absorbed inconsistently. The newer microemulsion version (Neoral) fixed that - but now, even among microemulsion generics, absorption varies. One study found only 41.7% of generic manufacturers provide detailed bioequivalence data to doctors. That means your pharmacist might switch you to a new generic without your transplant team even knowing. And if your levels drop, it’s not because you missed a dose - it’s because the pill you just took is chemically different. The European Medicines Agency warned in 2020: switching between different generic tacrolimus products without monitoring can lead to rejection or toxicity. The FDA doesn’t require interchangeability studies for these drugs. They’re approved as “equivalent,” not “interchangeable.” That’s a legal loophole with real consequences.
What Transplant Centers Are Doing About It
Most major transplant centers now have strict rules. If you’re on a generic, they want you to stay on the same one - from the same manufacturer - forever. Many hospitals now sign contracts with a single generic supplier to avoid switching. Over two-thirds of transplant programs adopted this “single-source” strategy by 2023. When a switch happens - whether forced by insurance or chosen by a patient - the protocol is intense. Blood levels are checked weekly for the first month. Dose adjustments are common. Patients are told to take their pills at the same time every day, within one hour. No grapefruit. No St. John’s wort. No changes to diet or other meds without approval. Transplant pharmacists are now trained to track not just the drug name, but the manufacturer. A prescription for “tacrolimus” isn’t enough. It has to say “tacrolimus [Manufacturer X].” That’s how they prevent accidental switches.What You Need to Know If You’re on a Generic
If you’re taking generic cyclosporine or tacrolimus, here’s what you need to do:- Always ask your pharmacist: Which manufacturer is this? Write it down.
- If your prescription changes - even if it’s still called “tacrolimus” - call your transplant team before you fill it.
- Don’t switch between generics just because your insurance says so. Push back. Ask for the one you’ve been stable on.
- Keep a log of your blood levels and any new side effects: tremors, headaches, nausea, changes in urine output.
- Never stop or skip doses, even if you feel fine. Rejection doesn’t always have symptoms.