When you pick up a prescription at the pharmacy, you might see a name like atorvastatin instead of Lipitor, or metformin instead of Glucophage. These are generic drugs-medications that work just like their brand-name versions but cost far less. But behind those simple names lies a complex system designed to organize, regulate, and guide how drugs are used. Understanding how generic drugs are classified isn’t just for pharmacists or doctors. It affects your prescriptions, your insurance costs, and even your safety.
Therapeutic Classification: What the Drug Treats
The most common way to group generic drugs is by what condition they treat. This is called therapeutic classification. Think of it as sorting medicines by their job. If a drug lowers blood pressure, it’s a cardiovascular agent. If it kills cancer cells, it’s an antineoplastic. This system is used in nearly every hospital and clinic in the U.S. because it’s practical. Doctors don’t need to know the chemical structure-they need to know what the drug does for the patient.
The FDA and USP maintain the official therapeutic categories, which break down into broad groups like:
- Analgesics (pain relievers)
- Antibiotics (infection fighters)
- Antihypertensives (blood pressure drugs)
- Antidepressants
- Antidiabetics
- Endocrine agents (hormone regulators)
Each of these has subcategories. For example, under analgesics, you’ll find non-opioid painkillers like acetaminophen and opioid ones like morphine. Under antihypertensives, you’ll see ACE inhibitors, beta-blockers, and calcium channel blockers. The system is detailed enough to handle over 300 categories, according to the American Hospital Formulary System.
But here’s the catch: some drugs do more than one thing. Aspirin, for instance, reduces pain, lowers fever, and thins blood. Should it be in analgesics or anticoagulants? The current system picks one primary use, which can cause confusion. That’s why the FDA introduced Therapeutic Categories Model 2.0 in 2023-this new version lets drugs have a main category and one or two secondary ones. It rolls out fully by January 2025.
Pharmacological Classification: How the Drug Works
While therapeutic classification asks, “What does this drug treat?”, pharmacological classification asks, “How does it work?” This is the science behind the medicine. It groups drugs by their biological mechanism-what protein, receptor, or enzyme they target.
For example:
- Drugs ending in -lol (like metoprolol, propranolol) are beta-blockers-they block adrenaline receptors to slow the heart.
- Drugs ending in -prazole (like omeprazole, esomeprazole) are proton pump inhibitors-they shut down acid production in the stomach.
- Drugs ending in -sartan (like losartan, valsartan) are angiotensin II receptor blockers-they relax blood vessels.
The U.S. Pharmacopeia recognizes 87 of these naming stems, and they’re not just for show. Studies show they reduce medication errors by 18%. If you see a drug with -tinib at the end, you know it’s a kinase inhibitor-likely used for cancer. This system is essential for researchers and pharmacists, but it’s less useful for patients or general practitioners because it requires deeper knowledge of biology.
There are over 1,200 distinct pharmacological classes. Some are very specific, like “BRAF V600E Inhibitors,” used only for certain melanoma cases. Others are broad, like “Diuretics,” which includes drugs that work in different parts of the kidney but all increase urine output. The challenge? A drug might belong to multiple pharmacological classes. Duloxetine, for example, affects both serotonin and norepinephrine, making it an antidepressant and a pain reliever at the same time. That’s why many doctors get confused between therapeutic and pharmacological groupings.
DEA Schedules: Legal Control and Abuse Risk
Not all drugs are created equal under the law. The Drug Enforcement Administration (DEA) classifies controlled substances into five schedules based on their potential for abuse and medical use. This system was created in 1970 under the Controlled Substances Act and still governs who can prescribe, how prescriptions are written, and how pharmacies store these drugs.
Here’s how the schedules break down:
- Schedule I: No accepted medical use, high abuse potential. Examples: heroin, LSD, marijuana (though this is changing).
- Schedule II: High abuse potential, but accepted medical use. Examples: oxycodone, fentanyl, Adderall, methadone.
- Schedule III: Moderate abuse potential, accepted medical use. Examples: ketamine, buprenorphine, some forms of hydrocodone with acetaminophen.
- Schedule IV: Low abuse potential. Examples: Xanax, Valium, tramadol.
- Schedule V: Very low abuse potential. Examples: cough syrups with small amounts of codeine (under 200mg per 100ml).
These schedules affect your access. A Schedule II drug like oxycodone requires a written prescription (no refills), while a Schedule IV drug like alprazolam can be called in and refilled up to five times. The problem? The system doesn’t always match the science. Marijuana is still Schedule I in the U.S. federal system, even though it’s legal for medical use in 38 states and has FDA-approved derivatives like dronabinol (Schedule II). Critics say this creates a dangerous gap-researchers can’t study it easily, but patients are using it anyway.
Insurance Tiers: What You Pay Out of Pocket
Insurance companies don’t care about therapeutic or pharmacological categories-they care about cost. That’s why they use tiered formularies. Most plans have five tiers, and where your generic drug lands determines how much you pay.
Here’s a typical breakdown:
- Tier 1: Preferred generics-cheapest. Usually $5-$10 for a 30-day supply. This is where 75% of generic drugs land.
- Tier 2: Non-preferred generics-slightly more expensive, maybe $15-$25. Often because the insurer has a deal with a different brand.
- Tier 3: Preferred brand-name drugs-costs 25-35% more than Tier 2 generics, even if the active ingredient is identical.
- Tier 4: Non-preferred brand-name drugs-high cost, often requiring prior authorization.
- Tier 5: Specialty drugs-expensive biologics, cancer treatments, rare disease meds. Can cost hundreds or thousands.
Here’s the kicker: two identical generic drugs-same active ingredient, same dosage, same manufacturer-can be on different tiers just because of a contract between the insurer and the drug distributor. Pharmacists report that 43% of prior authorization requests come from tier disputes, not medical necessity. A patient might get a generic for high blood pressure that’s on Tier 2, but their doctor switches them to a different generic that’s on Tier 3-costing them $20 more a month. No clinical reason. Just paperwork.
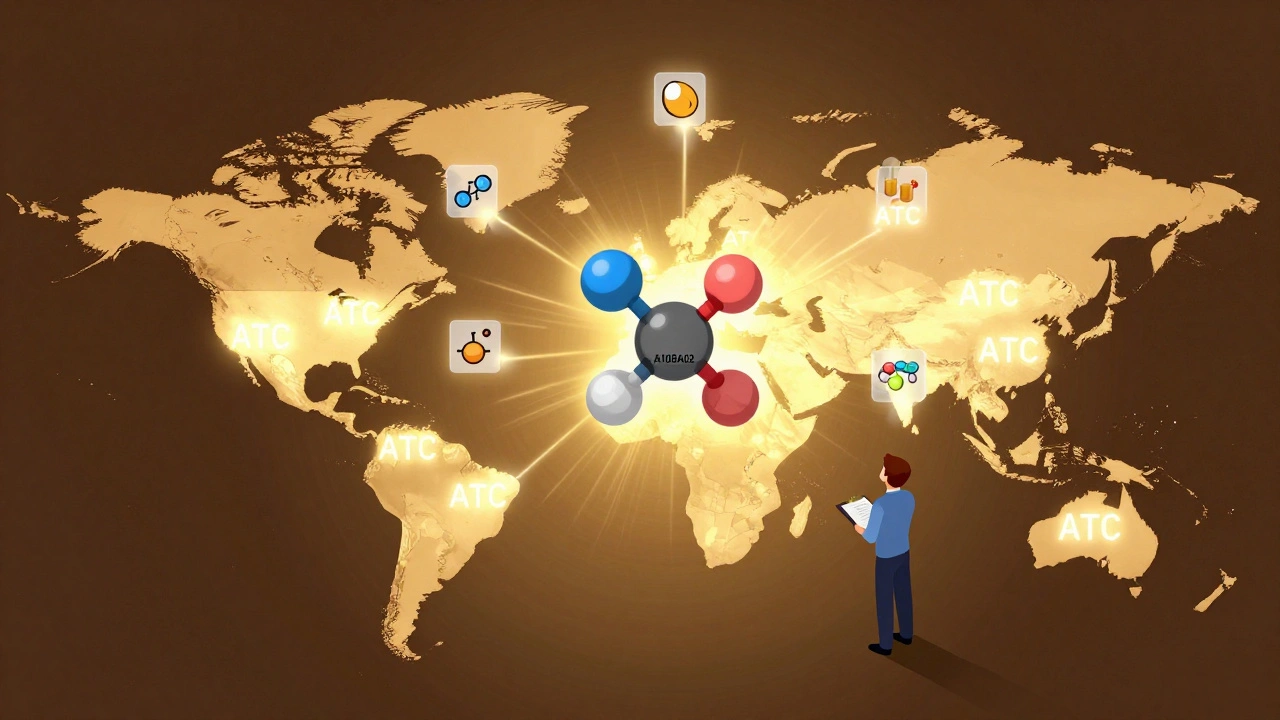
The ATC System: The Global Standard
While the U.S. uses multiple systems, the rest of the world mostly follows the World Health Organization’s Anatomical Therapeutic Chemical (ATC) classification. It’s a five-level code that tells you exactly what a drug is:
- Level 1: Anatomical group (e.g., A = Alimentary tract and metabolism)
- Level 2: Therapeutic subgroup (e.g., A10 = Drugs used in diabetes)
- Level 3: Pharmacological subgroup (e.g., A10B = Blood glucose lowering drugs, excluding insulins)
- Level 4: Chemical subgroup (e.g., A10BA = Biguanides)
- Level 5: Chemical substance (e.g., A10BA02 = Metformin)
The ATC system is used in 143 countries and tracks over 5,000 substances. It’s precise, consistent, and updated every quarter. In 2022 alone, 217 new drugs got ATC codes. It’s the gold standard for global drug research, public health reporting, and pharmacy data systems. The U.S. doesn’t use it officially, but many hospitals and research institutions do-especially for international studies.
Why Classification Matters to You
These systems aren’t just bureaucratic noise. They directly affect your health and wallet.
If your doctor prescribes a drug without knowing its classification, they might accidentally pick one that interacts badly with another. For example, combining a beta-blocker (like metoprolol) with a calcium channel blocker (like diltiazem) can slow your heart too much. Classification helps avoid those mistakes.
Insurance tiers can make you switch drugs mid-treatment. If your Tier 1 generic runs out of stock, your insurer might push you to a Tier 2 version that’s less effective for you. You might not even know why your blood sugar or blood pressure changed.
And if you’re on a controlled substance, the DEA schedule determines how often you can refill, whether you need a new prescription every month, and if your pharmacy can dispense it without special approval.
Understanding these systems helps you ask better questions. If your pharmacy says your generic is now $15 instead of $5, ask: “Is this a different tier?” If your doctor says you need a new medication, ask: “Is this the same pharmacological class as what I was on?” You don’t need to memorize all the categories-but knowing they exist makes you a smarter patient.
What’s Changing in 2025
Drug classification is evolving fast. The FDA’s new Therapeutic Categories Model 2.0 will let drugs have multiple primary uses. The DEA is under pressure to reclassify marijuana-possibly moving it to Schedule III, which would open the door to more research and insurance coverage.
AI tools like IBM Watson Health’s Drug Insight platform are starting to predict the best classification for new drugs with over 92% accuracy. And with 78% of new drugs in development having multiple mechanisms of action, the old one-size-fits-all systems are cracking.
By 2028, experts predict we’ll need hybrid classification systems that combine therapeutic use, pharmacological action, and even genetic markers. That’s the future of precision medicine.
For now, the current systems work-but they’re messy. The key is knowing which one applies when. Therapeutic for treatment. Pharmacological for science. DEA for legality. Insurance tiers for cost. ATC for global context. And always, always ask why a drug is being chosen-not just what it is.
What’s the difference between generic and brand-name drug classifications?
There’s no difference in how they’re classified. Generic and brand-name drugs with the same active ingredient are grouped the same way-therapeutically, pharmacologically, and legally. The classification is based on the molecule, not the brand. A generic atorvastatin is in the same category as Lipitor: a statin (therapeutic), HMG-CoA reductase inhibitor (pharmacological), and not controlled (DEA). The only difference is cost and packaging.
Why are some generic drugs more expensive than others?
It’s not about the drug-it’s about insurance tiers. Two identical generic drugs can be priced differently because insurers negotiate deals with distributors. One might be on Tier 1 (low cost), another on Tier 2 (higher cost), even if they’re made by the same company. Always check your plan’s formulary or ask your pharmacist: “Is this the preferred generic?”
Can a drug be in more than one classification system?
Yes, absolutely. A single drug can belong to multiple categories at once. For example, duloxetine is classified as an antidepressant (therapeutic), a serotonin-norepinephrine reuptake inhibitor (pharmacological), and it’s not controlled (DEA). The ATC code even breaks it down further. Classification systems are layered-they serve different purposes, so overlap is normal and expected.
How do I know if my drug is a controlled substance?
Check the label. Controlled substances are clearly marked with a “C” followed by a number (e.g., C-III). You can also ask your pharmacist or look up the drug on the DEA’s website. If you’re prescribed a painkiller, sleep aid, stimulant, or anti-anxiety medication, there’s a good chance it’s controlled. Always follow refill rules-exceeding them can be illegal.
Do generic drugs have different side effects because of classification?
No. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and performance as the brand. Side effects are identical. Differences in inactive ingredients (like fillers or dyes) can rarely cause reactions, but that’s not related to classification. Classification tells you what the drug does-not how your body reacts to it.
